Express LD Iliac and Biliary Metallic Stent
Order Product Form


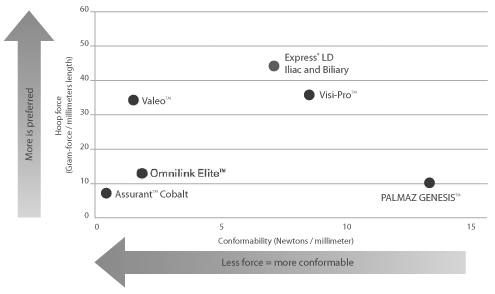
Compression resistance and conformability are crucial stent attributes that help improve vessel patency within tortuous vasculature.
Compression Resistance measured as the force required to compress the fully expanded stent. Compression Resistance measured using a hoop force tester in 37° air. Conformability measured by torque required to achieve a constant curvature target as measured by angular deflection.
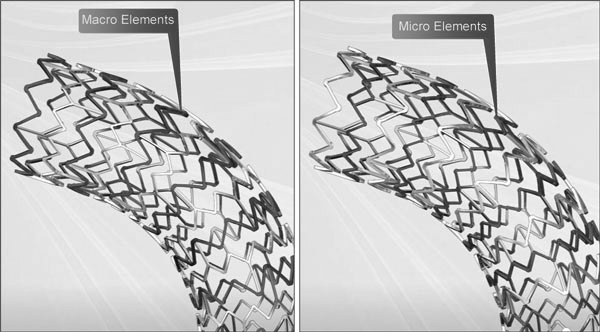
Express LD Stent System features a patented Tandem Architecture Stent Design
Micro Elements engineered to provide:
- Flexibility during placement
- Conformability upon deployment
- Maximum apposition
Macro Elements engineered for:
- Balanced compression resistance
- Strength—even in arterial bifurcations
Clinical Information
MELODIE Trial Summary
MELODIE is a prospective, multi-center, single arm study to obtain additional data on the safety and efficacy of the Express Vascular LD stent implantation in the treatment of stenosed or occlusive atherosclerotic disease (de novo or restenotic) in iliac arteries (common or external).
MELODIE Trial Objective
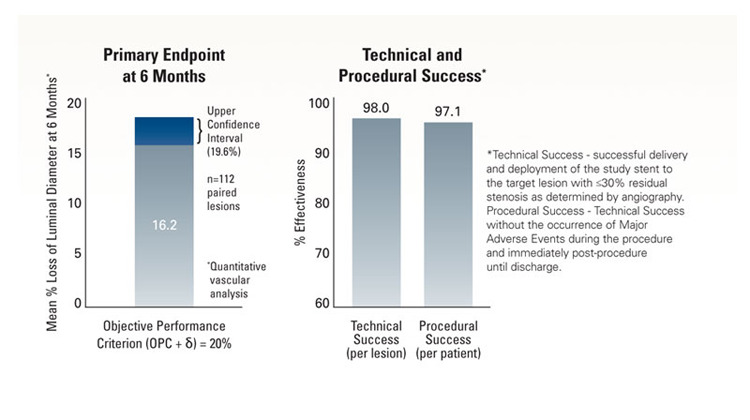
The objective was to demonstrate non-inferiority of the Express LD Stent for the treatment of atherosclerotic iliac artery lesions as compared to an objective performance criterion (OPC), with a primary endpoint of mean percent luminal diameter loss at 6 months.
Primary Endpoint
Definition
- Angiographic mean percent loss of luminal diameter at 6 months post-procedure, defined as [POST MLD1 - FUP MLD2 / POST MLD] x 100
1Post MLD = Post-procedure minimum lumen diameter.
2FUP MLD = Follow-up minimum lumen diameter at 6 months.
Outcome
- Mean percent luminal diameter loss at 6 months = 16.2% ± 18.4%
- Upper Confidence Int = 19.6%
- Clinical follow-up rate at 6 months = 92.6% (138/150)
- This rate was non-inferior to the Objective Performance Criterion = δof 20%
24-Month Results
-
The Express LD Stent = 8.9%, 10.2% TLR at 1 and 2 years and no device/procedure-related death
-
Sustained Stent Target Lesion Patency measured by CTA with 97.2% at 1 year and 94.1% at 2 years
| Product Code | - |
| Application | Iliac and Biliary Premounted Stent System |
| Manufacturer Country | USA |
| Guarantee Type | Based on manufacturer recommendations |